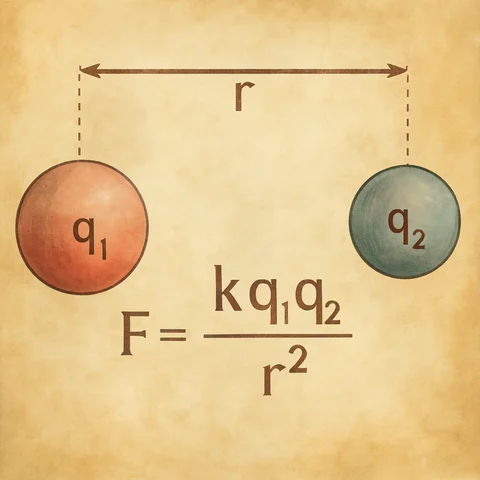The electrostatic force and Coulomb’s law online simulations on this page allow us to visualize how this important force of nature is generated. We will study how its value is calculated from Coulomb’s Law.
What is electrostatic force
Electrostatic force is a fundamental force in nature that arises from the interaction between electric charges. It is a force of an electrical nature that acts at a distance and can be attractive or repulsive, depending on the properties of the charges involved.
Formula of Coulomb’s law
Electrostatic force is governed by Coulomb’s Law, formulated by the French physicist Charles-Augustin de Coulomb. According to this law, the magnitude of the electrostatic force between two point charges is directly proportional to the product of their charge magnitudes and inversely proportional to the square of the distance separating them. Mathematically, the formula of Coulomb’s law is expressed as:
Electrostatic force = (k x q₁ x q₂) / r².
Where:
Electrostatic force is the magnitude of the force between charges.
k is the Coulomb constant, which depends on the medium in which the charges are located and has a value of approximately 9 × 10^9 N-m²/C² in vacuum.
q₁ and q₂ are the magnitudes of the charges involved.
r is the distance between the charges.
Electrostatic force applications
The electrostatic force is fundamental to many aspects of physics and has numerous applications in everyday life and technology. For example, it is responsible for the interaction between electrons and nuclei in atoms, maintaining the stability of matter. It also determines the structure and properties of chemical compounds.
In the field of electricity and electronics, electrostatic force is essential for the operation of electrical devices, such as capacitors, which store energy in the form of charges separated by a potential difference. It is also the force behind electrostatic phenomena, such as the attraction of charged objects by rubbing or the repulsion between electrically charged balloons.
Electrostatic force is also applied in technologies such as electrodynamics, electricity generation and distribution, and touch screen technology. In addition, it has implications in fields such as astrophysics, where electrostatic interactions are studied in the behavior of stars and galaxies.
Electrostatic force and Coulomb's law simulations
- Force
- Coulomb I
- Coulomb II
Giants of science
“If I have seen further, it is by standing on the shoulders of giants”
Isaac Newton

James Clerk Maxwell
–

Michael Faraday
–
Become a giant


Principles of Modeling, Simulations, and Control for Electric Energy Systems



Principles of Electric Circuits | 电路原理



Electrotechnique I



Electromagnetic Compatibility Essentials



AP® Physics 1: Challenging Concepts



AP® Physics 1 – Part 1: Linear Motion



AP® Physics 2: Challenging Concepts



AP® Physics 1

Professional development for Educators


Teaching Science and Engineering



Higher education teaching in the age of AI



Assessment Design with AI



STEM Outside