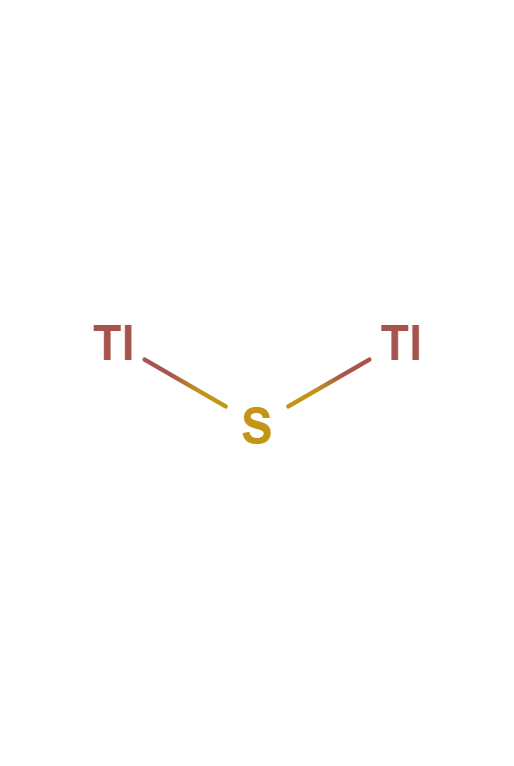
The online chemical salt solubility simulations on this page allow you to interactively visualize the dissolution process of a salt. In addition, the molecules of Sodium Chloride, Copper Iodide and Thallium Sulfide are also shown.
What are chemical salts?
Chemical salts are ionic compounds formed from the combination of a cation with an anion.
Solubility of chemical salts
The solubility of a salt depends on its chemical structure and the environmental conditions in which it is found, such as temperature and pressure. The solubility of salts is of great importance in many chemical processes and in industry.
The solubility of a salt can be determined by a number of experiments. In general, the solubility of salts increases with temperature. As the temperature increases, the solvent molecules move faster, allowing for greater interaction with the salt ions and greater dissolution. However, there are some exceptions where solubility decreases with temperature.
Factors affecting the solubility of the chemical salts
In addition to temperature, the pH of the medium can also affect the solubility of a salt. Some salts are more soluble in acidic media, while others are more soluble in basic media. This is because the pH of the medium can influence the charge of the salt ions, which in turn affects their ability to interact with the solvent.
Importance of chemical salts solubility
The solubility of salts is also of great importance in industry. For example, in the manufacture of pharmaceuticals, it is important that the active ingredients dissolve easily in the body so that they can be effective. In food production, the solubility of salts can affect the texture and appearance of the final products.
In short, these online chemical salt simulations will help you to better understand what these important chemical compounds are and how they work.
Simulations and 3d molecules of chemical salts
- Solubility
- Sodium
- Copper
- Thallium
Salts and solubility
Add different salts to water, and then watch them dissolve and achieve dynamic equilibrium with the solid precipitate. Compare the number of ions in the highly soluble NaCl solution with other poorly soluble salts. Relate the charges on the ions to the number of ions in the formula of a salt. Calculate the Ksp values.
Sodium Chloride
Sodium chloride, common salt or table salt, referred to in its mineral form as halite, is a chemical compound with the formula NaCl. Sodium chloride is one of the salts responsible for the salinity of the ocean and the extracellular fluid of many organisms. It is also the component of common salt, used as a seasoning and food preservative.
Sodium Chloride
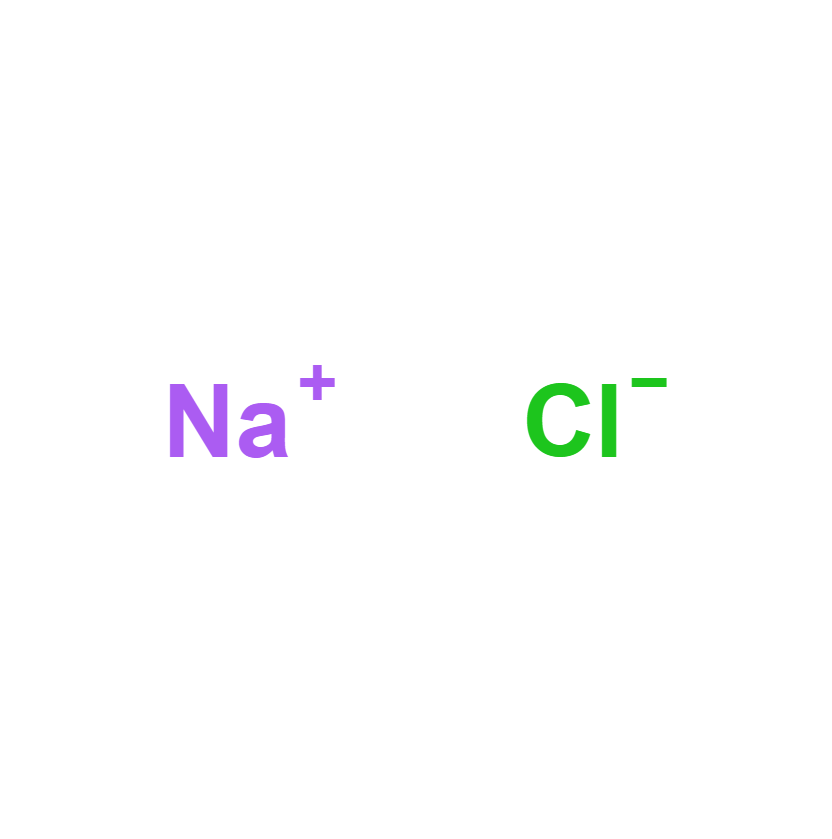
 Single bond
Single bond
Double bond
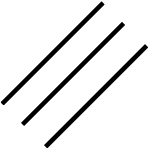 Triple bond
Triple bond
Wedge bond

Hash bond
Copper(I) Iodide
Copper(I) iodide is the inorganic compound with the formula CuI. It is also known as cuprous iodide. It is useful in a variety of applications ranging from organic synthesis to cloud seeding.
Copper(I) Iodide
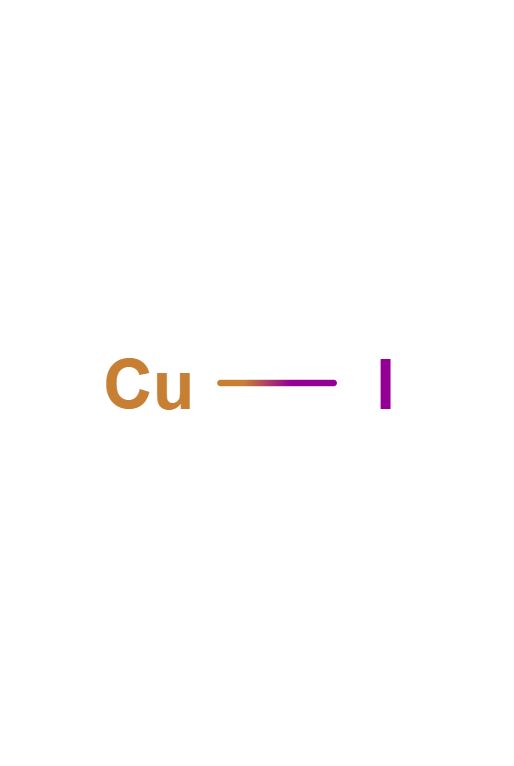
 Single bond
Single bond
Double bond
 Triple bond
Triple bond
Wedge bond

Hash bond
Giants of science
“If I have seen further, it is by standing on the shoulders of giants”
Isaac Newton

Fritz Haber
–

Marie Curie
–
Become a giant


Introduction to Solid State Chemistry



Big Bang and the Origin of Chemical Elements



Preparing for CLEP Chemistry: Part 1



Pre-University Chemistry