Atomic model of hydrogen
How do scientists determine the structure of atoms, without looking at them? Test the different models by shooting light at the atom. See how the atomic model of hydrogen prediction matches the experimental results.
Hydrogen molecule
Hydrogen is the chemical element of atomic number 1, represented by the symbol H. It usually occurs in its molecular form. , The hydrogen molecule is made up of two hydrogen atoms, which gives rise to the diatomic gas H2 under normal conditions. This gas is flammable, colorless, odorless, non-metallic and insoluble in water.
Hydrogen
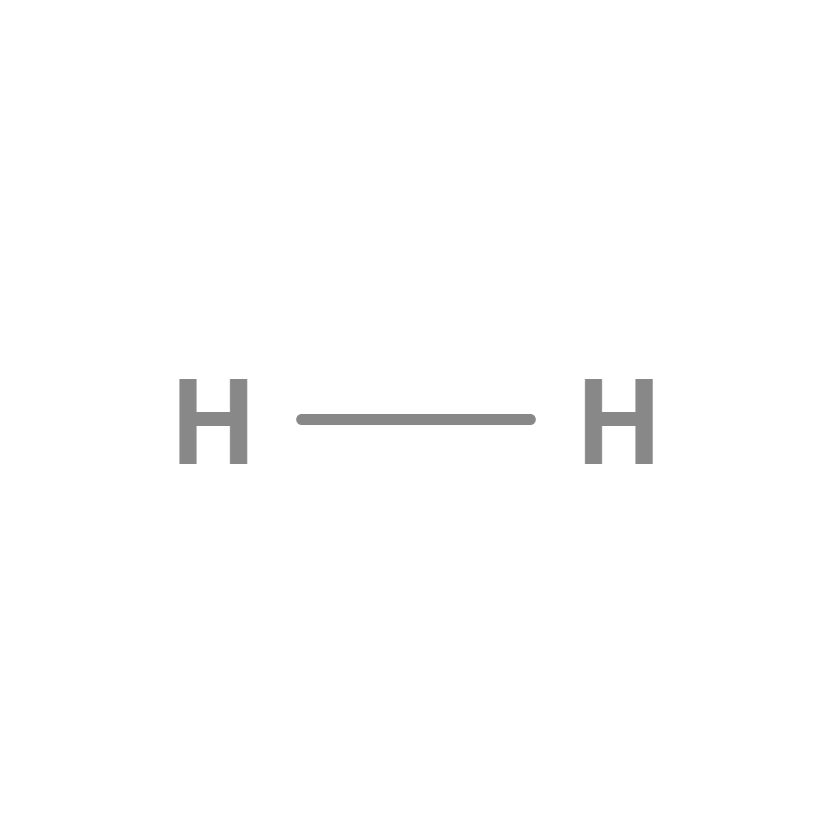
 Single bond
Single bond
Double bond
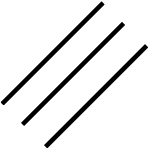 Triple bond
Triple bond
Wedge bond

Hash bond
- Atom
Models of the hydrogen atom
How do scientists determine the structure of atoms, without looking at them? Test the different models by shooting light at the atom. See how the model prediction matches the experimental results.
This Java simulation cannot run on this device because it has a screen that is too narrow. We recommend that, for a better user experience, you run it on a device with a wider screen.
Although this Java simulation can be run on your device, we recommend that for the better user experience, you run it on a device with a wider screen.
Giants of science
“If I have seen further, it is by standing on the shoulders of giants”
Isaac Newton

Gilbert Newton Lewis
–

Fritz Haber
–
Become a giant


Introduction to Computational Materials Design



From Atoms to Materials: Predictive Theory and Simulations



Digital Biomaterials



Cement Chemistry and Sustainable Cementitious Materials



Preparing for CLEP Chemistry: Part 1



Big Bang and the Origin of Chemical Elements



Pre-University Chemistry

Professional development for Educators


Teach computing: Physical computing with Raspberry Pi and Python



The Science of Learning – What Every Teacher Should Know



Teach teens computing: Programming in Python



Assessment Design with AI
































