The online 3D alkane hydrocarbon molecules of this page visualize through 3D images of the molecules of some of the most important hydrocarbons of this type: methane, ethane, propane and butane. We will discover what are alkane hydrocarbons, how is their nomenclature and formulation, as well as their presence in nature and their applications
What are alkane hydrocarbons
Alkane hydrocarbons are a class of fundamental organic compounds consisting of carbon and hydrogen atoms only. They are known as saturated hydrocarbons because all the bonds between the carbon atoms are single, meaning that they are saturated with hydrogen atoms.
Nomenclature and formulation of alkane hydrocarbons
These compounds are named according to the number of carbon atoms present in their molecule. For example, methane has only one carbon atom, ethane has two carbon atoms, propane has three carbon atoms and so on.
Their chemical structure is based on a linear or branched carbon chain, where hydrogen atoms are linked to carbon atoms by single bonds. The general formula for alkanes is CnH2n+2, where “n” represents the number of carbon atoms.
Presence of alkane hydrocarbons in nature
Alkanes can be found in different states of matter, from gases such as methane to liquids such as pentane and hexane, and even solids such as heptane. Alkane hydrocarbons are widely found in nature, forming a major part of natural gas deposits, where short-chain hydrocarbons such as methane, ethane, propane and butane predominate. Long-chain alkanes are mainly present in petroleum. They are also generated biologically in oxygen-depleted environments, such as swamps or the seabed, where methane is produced by bacteria. They can also be found in small quantities in living organisms, as part of plant waxes and certain oils. This diversity of origins underlines the relevance of alkanes both industrially and ecologically.
Applications of alkane hydrocarbons
Alkanes are widely used as fuels. Natural gas, which contains mainly methane, is an important energy source used for heating, electricity generation and as a fuel in vehicles. Gasoline, which is a mixture of hydrocarbons, also contains alkanes, such as heptane and octane, which provide energy for internal combustion engines.
In addition to their use as fuels, alkanes have applications in the petrochemical industry. They are used as feedstock for the production of plastics, such as polyethylene and polypropylene, as well as for the manufacture of chemicals, lubricants, solvents and detergents.
In summary, these online 3D alkane hydrocarbon molecules give you a visualization of the molecules that will undoubtedly help you to better understand these important chemical compounds. Don’t miss them!
Explore the exciting STEM world with our free, online, simulations and accompanying companion courses! With them you'll be able to experience and learn hands-on. Take this opportunity to immerse yourself in virtual experiences while advancing your education - awaken your scientific curiosity and discover all that the STEM world has to offer!
3D alkane hydrocarbon molecules
- Methane
- Ethane
- Propane
- Butane
- Enlaces
Methane
Methane is the simplest alkane hydrocarbon, whose chemical formula is CH4. Each of the hydrogen atoms is bonded to carbon by a covalent bond. It is a non-polar substance that occurs as a gas at ordinary temperatures and pressures. It is colorless, odorless and insoluble in water.
Methane
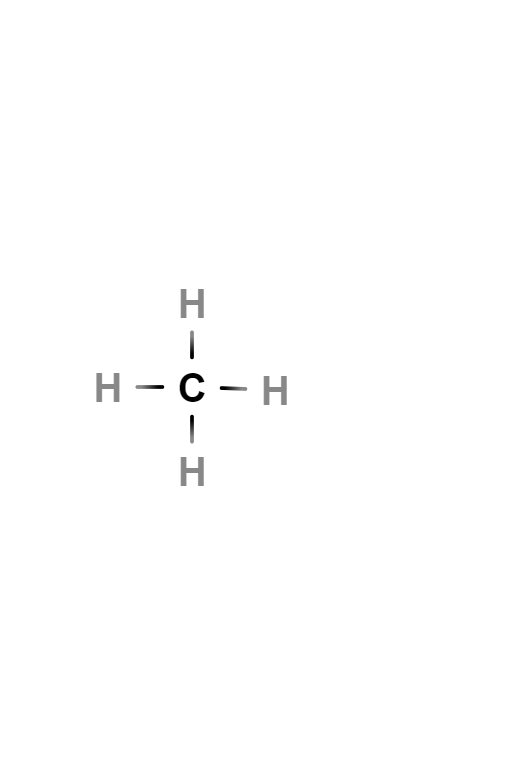
 Single bond
Single bond
Double bond
 Triple bond
Triple bond
Wedge bond

Hash bond
Ethane
Ethane is an aliphatic alkane hydrocarbon with two carbon atoms, formula C2H6. Under normal conditions it is gaseous and an excellent fuel. Its boiling point is -88 °C. It is found in appreciable quantities in natural gas.
Ethane
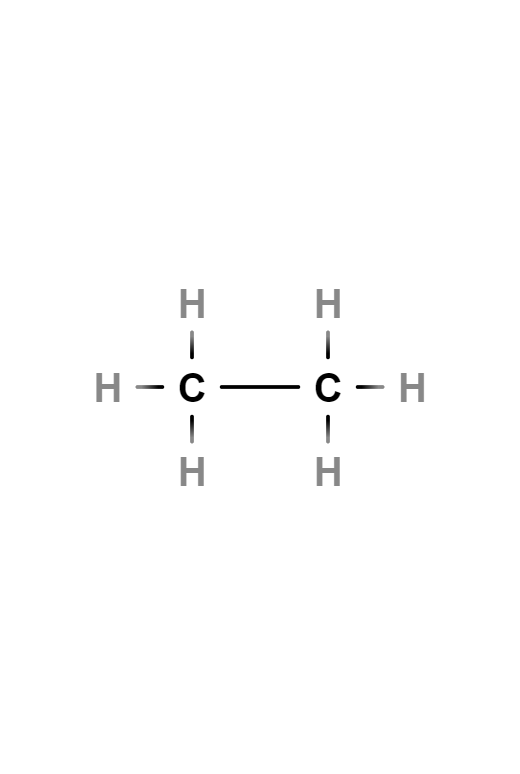
 Single bond
Single bond
Double bond
 Triple bond
Triple bond
Wedge bond

Hash bond
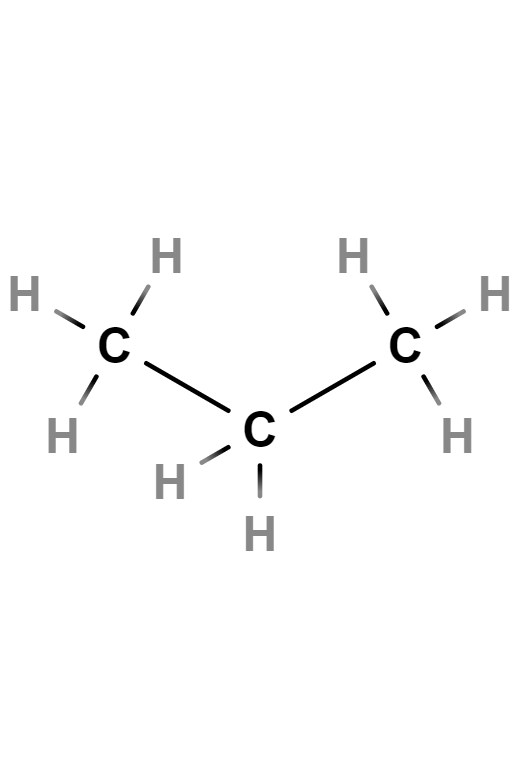
Propane
Propane is a colorless, odorless gas. It belongs to the aliphatic hydrocarbons with carbon single bonds, known as alkanes. Its chemical formula is C3H8.
Propane
 Single bond
Single bond
Double bond
 Triple bond
Triple bond
Wedge bond

Hash bond
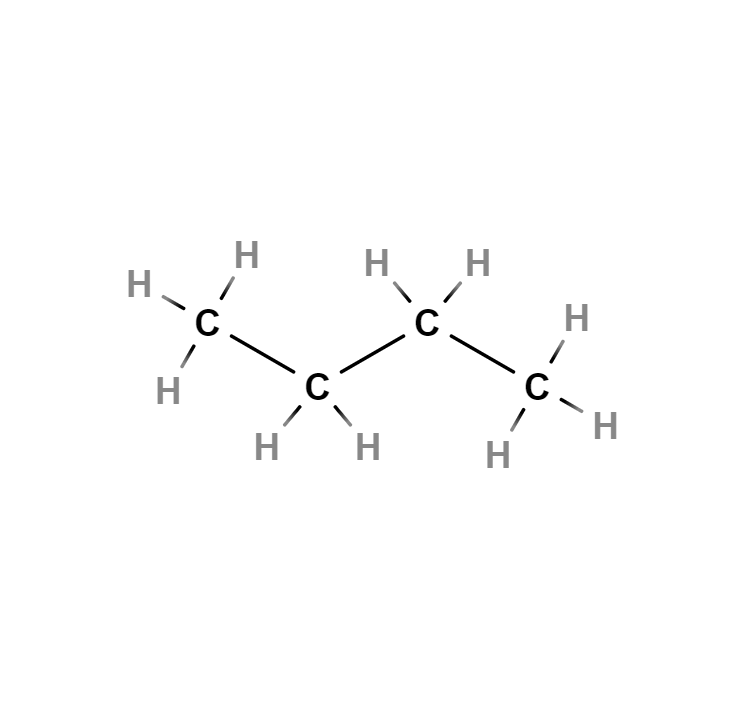
Butane
Butane, also called n-butane, is a saturated, paraffinic or aliphatic, flammable, gaseous hydrocarbon that liquefies at atmospheric pressure at -0.5 °C, consisting of four carbon atoms and ten hydrogen atoms, whose chemical formula is C4H10. An isomer of this gas can also be called by the same name: isobutane or methylpropane.
Butane
 Single bond
Single bond
Double bond
 Triple bond
Triple bond
Wedge bond

Hash bond
Giants of science
“If I have seen further, it is by standing on the shoulders of giants”
Isaac Newton

Friedrich Wöhler
–
Become a giant


Principles of Biochemistry



Medicinal Chemistry: The Molecular Basis of Drug Discovery



Introduction to Pharmacology



Biorefinery: From Biomass to Building Blocks of Biobased Products



Big Bang and the Origin of Chemical Elements



Preparing for CLEP Chemistry: Part 1



Pre-University Chemistry

























