3D alkane hydrocarbon molecules
- Methane
- Ethane
- Propane
- Butane
- Enlaces
Methane
Methane is the simplest alkane hydrocarbon, whose chemical formula is CH4. Each of the hydrogen atoms is bonded to carbon by a covalent bond. It is a non-polar substance that occurs as a gas at ordinary temperatures and pressures. It is colorless, odorless and insoluble in water.
Methane
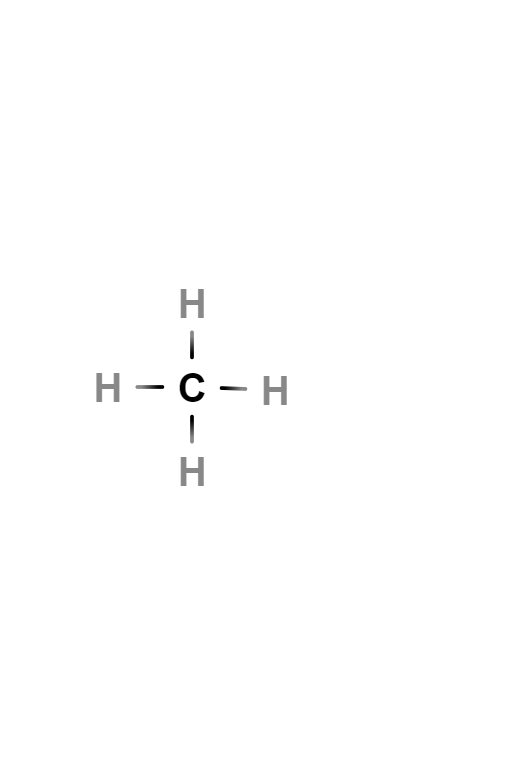
 Single bond
Single bond
Double bond
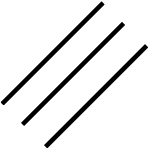 Triple bond
Triple bond
Wedge bond

Hash bond
Ethane
Ethane is an aliphatic alkane hydrocarbon with two carbon atoms, formula C2H6. Under normal conditions it is gaseous and an excellent fuel. Its boiling point is -88 °C. It is found in appreciable quantities in natural gas.
Ethane
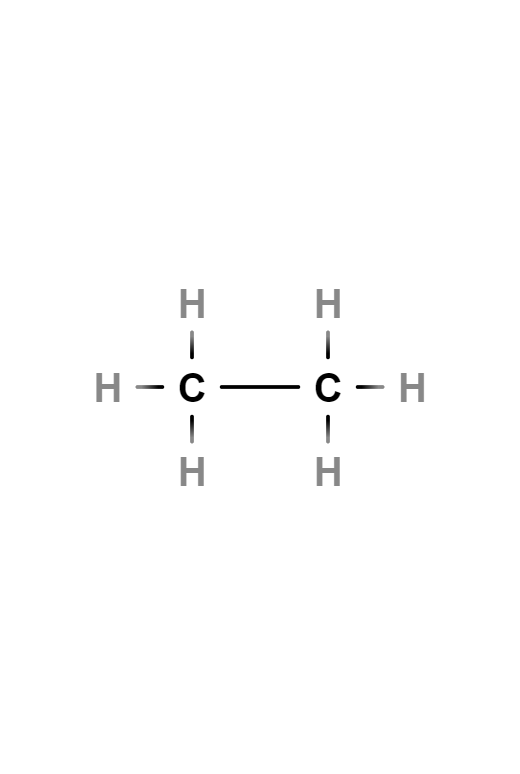
 Single bond
Single bond
Double bond
 Triple bond
Triple bond
Wedge bond

Hash bond
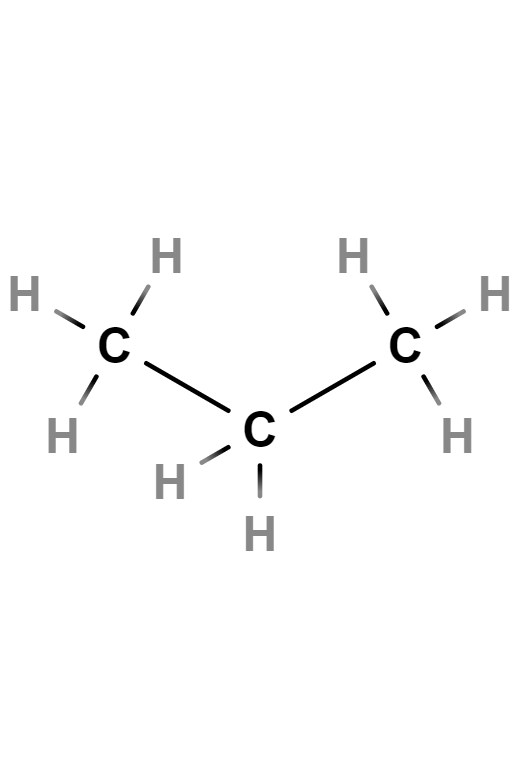
Propane
Propane is a colorless, odorless gas. It belongs to the aliphatic hydrocarbons with carbon single bonds, known as alkanes. Its chemical formula is C3H8.
Propane
 Single bond
Single bond
Double bond
 Triple bond
Triple bond
Wedge bond

Hash bond
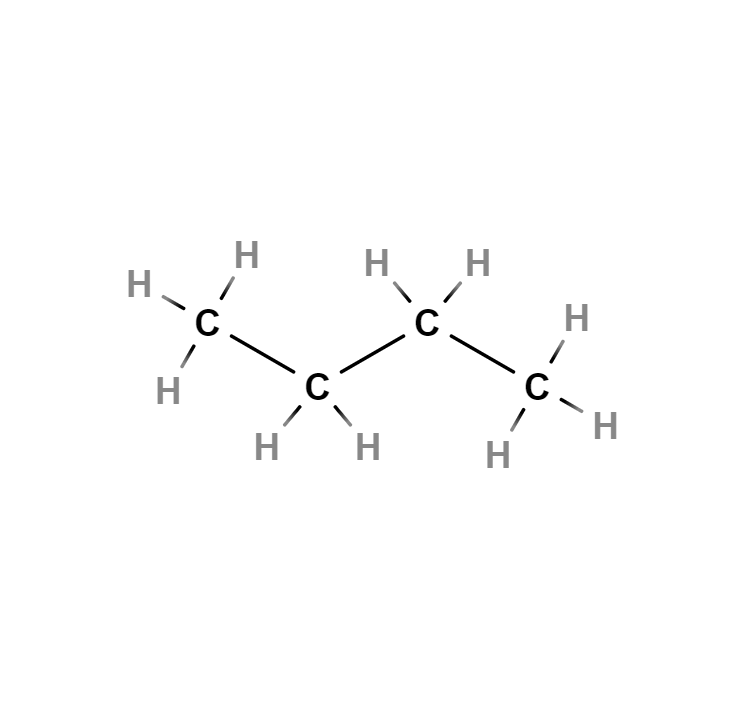
Butane
Butane, also called n-butane, is a saturated, paraffinic or aliphatic, flammable, gaseous hydrocarbon that liquefies at atmospheric pressure at -0.5 °C, consisting of four carbon atoms and ten hydrogen atoms, whose chemical formula is C4H10. An isomer of this gas can also be called by the same name: isobutane or methylpropane.
Butane
 Single bond
Single bond
Double bond
 Triple bond
Triple bond
Wedge bond

Hash bond
Covalent hydrocarbon bonds
This simulation allows us to build hydrocarbon molecules by combining carbon and hydrogen atoms.
Giants of science
“If I have seen further, it is by standing on the shoulders of giants”
Isaac Newton

Friedrich Wöhler
–
Become a giant


Principles of Biochemistry



Medicinal Chemistry: The Molecular Basis of Drug Discovery



Introduction to Pharmacology



Biorefinery: From Biomass to Building Blocks of Biobased Products



Preparing for CLEP Chemistry: Part 1



Big Bang and the Origin of Chemical Elements



Pre-University Chemistry

Professional development for Educators


Advancing Learning Through Evidence-Based STEM Teaching



Learn Like a Pro: Science-Based Tools to Become Better at Anything



HP AI Teacher Academy



Teach teens computing: Cybersecurity