The online greenhouse effect simulations on this page serve as a model of how the greenhouse effect works and show the mechanism of the greenhouse effect. They also show some of the main greenhouse gases.
What is the greenhouse effect
The greenhouse effect is a natural process in which certain gases in the Earth’s atmosphere retain part of the infrared radiation emitted by the Earth after receiving solar energy. This causes the atmosphere to warm, allowing the surface temperature to become suitable for life.
The scientific basis for the greenhouse effect has been supported by multiple lines of evidence, including direct measurements of gas concentrations in the atmosphere, studies of infrared radiation, and models of the greenhouse effect that simulate the behavior of the Earth in response to changes in greenhouse gas levels.
How the greenhouse effect works. Mechanism of the greenhouse effect
Basic mechanism of the greenhouse effect is as follows. Solar radiation reaching the Earth consists mainly of short-wave radiation, such as visible light and ultraviolet (UV) rays. Some of this radiation is reflected back into space by the Earth’s surface and clouds, while some is absorbed by the atmosphere, the oceans and the Earth’s surface.
The Earth’s surface, once heated by solar radiation, emits long-wave radiation, also known as infrared (IR) radiation. Some gases present in the atmosphere have the capacity to absorb and re-emit part of this infrared radiation.
Main greenhouse gases
The main greenhouse gases are water vapor (H2O), carbon dioxide (CO2), methane (CH4) or nitrous oxide (N2O) have the ability to absorb and re-emit part of this infrared radiation. These gases are known as greenhouse gases because of their ability to trap and retain heat in the atmosphere. When infrared radiation is absorbed by greenhouse gases, the molecules become energized and subsequently emit radiation back in all directions, including towards the earth’s surface. This results in a net increase in thermal energy on Earth, leading to an increase in atmospheric and surface temperature.
Greenhouse effect simulations
- Model
- Radiation
- Water
- CO2
- Methane
- N2O
Greenhouse effect model
This simulation is a simple but very instructive model of the mechanism of the greenhouse effect. How do greenhouse gases affect the climate? Explore the atmosphere during the ice age and today. What happens when clouds are added? Change the concentration of greenhouse gases and see how the temperature changes. Then compare the effect of crystals. Zoom in and see how light interacts with molecules Do all gases in the atmosphere contribute to the greenhouse effect?
Radiation balance on Earth
This animation summarizes the various factors involved in the Earth’s radiation balance. Therefore, it is also a model of the greenhouse effect, albeit a very simplified one.
Water vapor
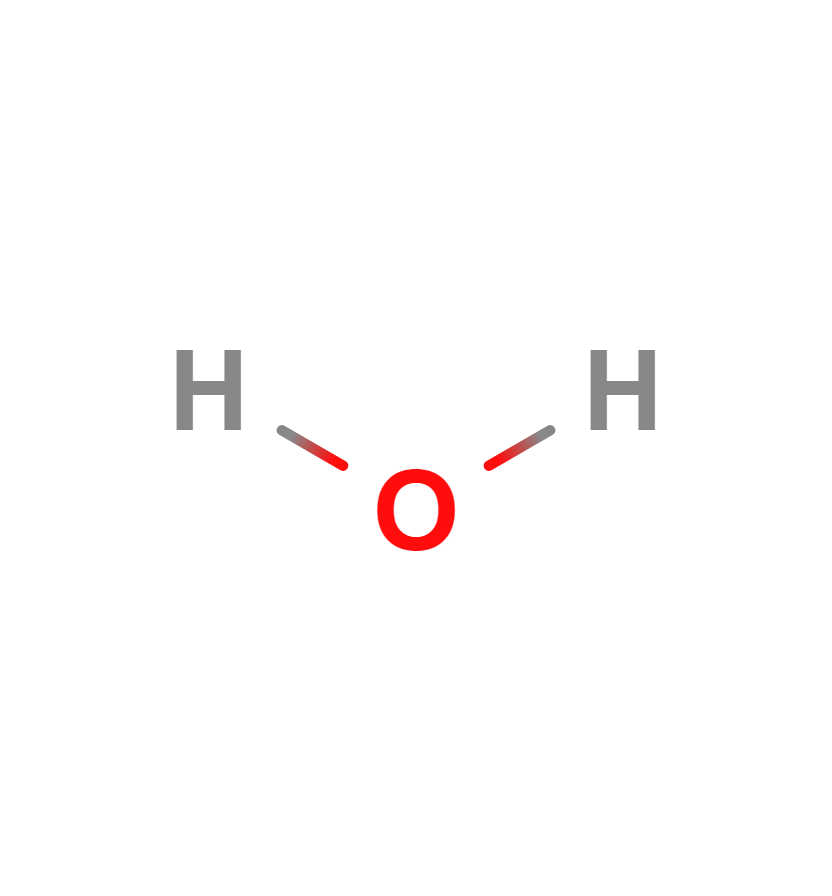
Water vapor is an important greenhouse gas. The water molecule is composed of two hydrogen atoms and one oxygen atom bonded together by a covalent bond. That is, the two hydrogen atoms and the oxygen atom are bonded together by sharing electrons. Its formula is H2O.
Water
 Single bond
Single bond
Double bond
 Triple bond
Triple bond
Wedge bond

Hash bond
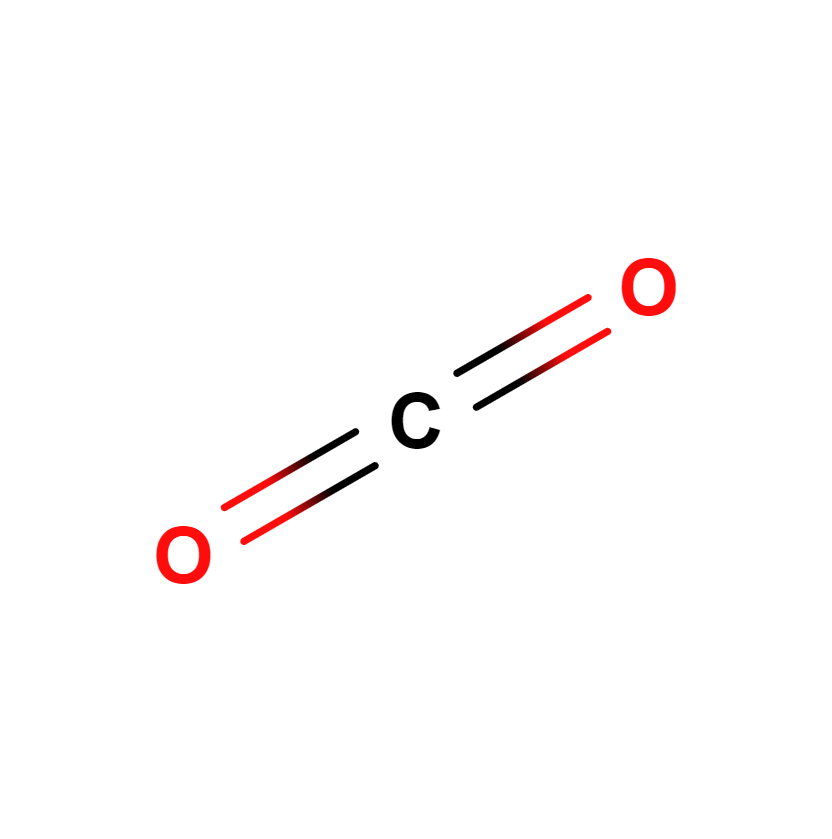
Carbon dioxide
Carbon dioxide, whose chemical formula is CO2, is a compound of carbon and oxygen that exists as a colorless gas at standard temperature and pressure conditions. Prior to the 2005 IUPAC standards, it was also known as carbonic anhydride.
Carbon dioxide
 Single bond
Single bond
Double bond
 Triple bond
Triple bond
Wedge bond

Hash bond
Methane
Methane is the simplest alkane hydrocarbon, whose chemical formula is CH4. Each of the hydrogen atoms is bonded to carbon by a covalent bond. It is a non-polar substance that occurs as a gas at ordinary temperatures and pressures. It is colorless, odorless and insoluble in water.
Methane
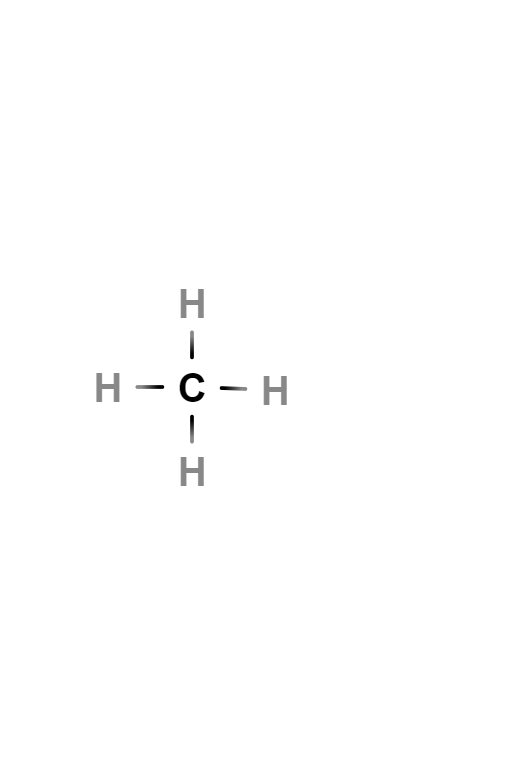
 Single bond
Single bond
Double bond
 Triple bond
Triple bond
Wedge bond

Hash bond
Nitrous oxide
Nitrous oxide, whose chemical formula is N2O, also known as laughing gas, is a colorless gas with a sweet, slightly toxic odor and anesthetic effect. It is one of the most important greenhouse gases and is a contributor to stratospheric ozone depletion.
Nitrous oxide
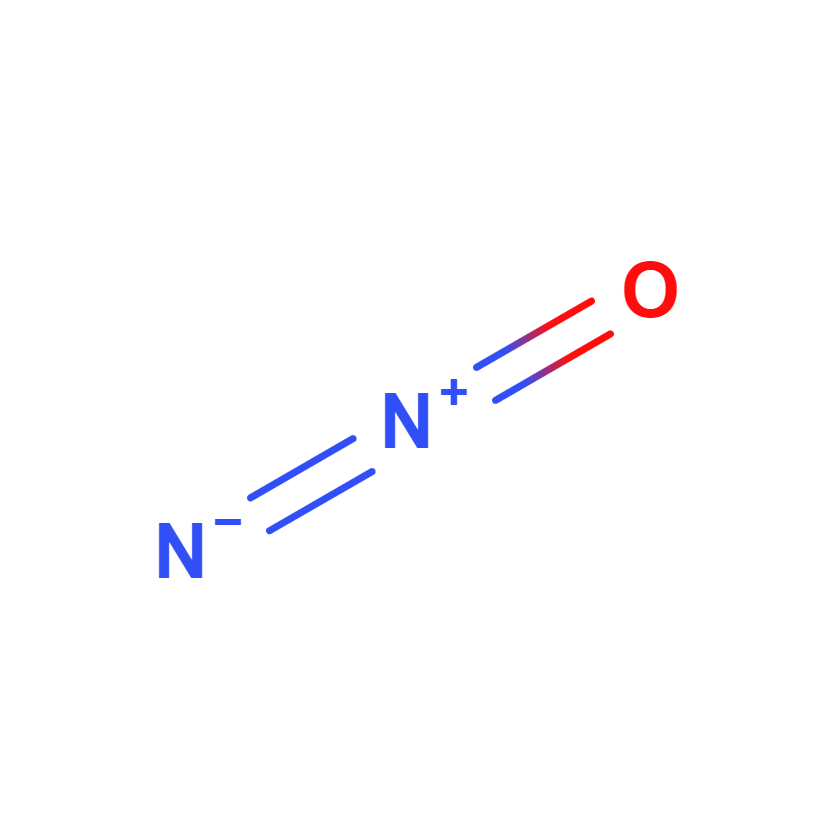
 Single bond
Single bond
Double bond
 Triple bond
Triple bond
Wedge bond

Hash bond
Giants of science
“If I have seen further, it is by standing on the shoulders of giants”
Isaac Newton

Luke Howard
–

Evangelista Torricelli
–
Become a giant


Modeling Climate Change



Introduction to Water and Climate



Global Warming Science



Climate Change: The Science and Global Impact



Sensing Planet Earth – Water and Ice



The History of Ancient Environments, Climate, and Life



Introduction to Deep Earth Science



Our Global Ocean – An Introduction Course

Professional development for Educators


Unlocking the Power of Generative AI with ChatGPT for Higher Education

























