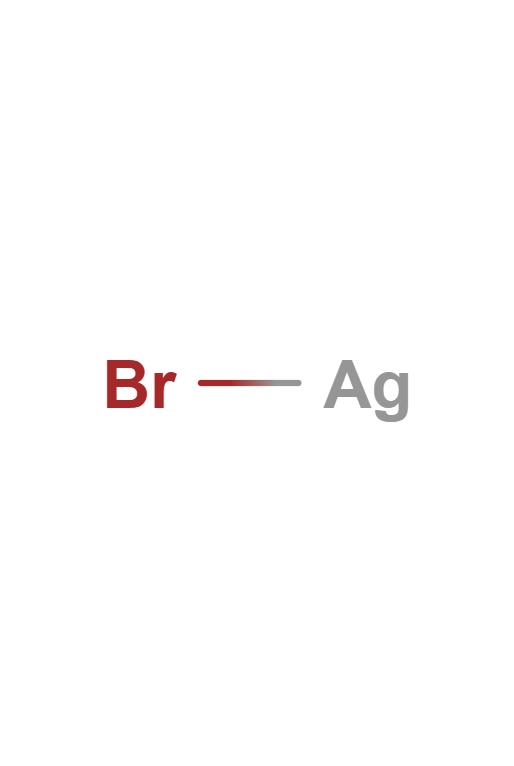Expand your knowledge about chemical salts, their properties, applications and formation process. The 3d molecules of chemical salts on this page shows what the molecules of Silver Arsenate, Mercuric Bromide, Strontium Phosphate, and Silver Bromide look like
What are chemical salts?
Chemical salts are ionic compounds formed from the combination of a cation with an anion. These compounds have a wide variety of uses in industry, medicine, agriculture and other fields.
Formation of chemical salts
Salts are formed through a chemical reaction between an acid and a base. During this reaction, the acid and base ions exchange charges to form a salt. For example, the combination of hydrochloric acid and sodium hydroxide produces sodium chloride (common salt) and water:
HCl + NaOH → NaCl + H2O.
Properties of chemical salts
Salts can have different properties, such as melting and boiling point, solubility, density and electrical conductivity. These properties depend on the chemical structure of the salt and the forces that exist between the ions in the crystal lattice.
Applications of chemical salts
Salts are used in a wide variety of applications. For example, sodium chloride is used as a seasoning and preservative in the food industry, as a flux in metallurgy and as a raw material in the production of other chemical compounds. Aluminum sulfate is used in water purification as a coagulant and flocculant to remove impurities. Ammonium nitrate is used as a fertilizer in agriculture and as an explosive in mining.
Salts also have applications in medicine. For example, potassium chloride is used to treat hypokalemia (low potassium in the blood) and magnesium sulfate is used as a laxative and in the treatment of eclampsia (complication of pregnancy).
3d molecules of chemical salts
- Silver
- Mercury
- Strontium
- Silver II
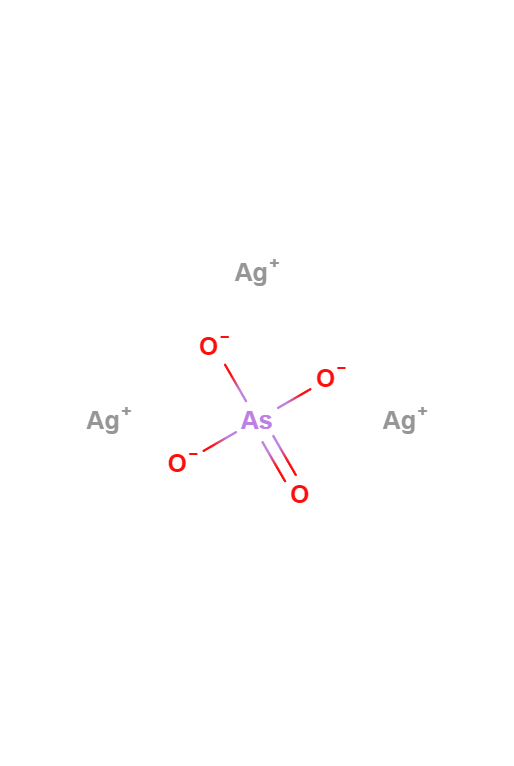
Silver Arsenate
Silver Arsenate is an inorganic compound with the formula Ag3AsO4. It has been used in qualitative analysis to distinguish between phosphate and arsenate solutions.
Silver arsenate
 Single bond
Single bond
Double bond
 Triple bond
Triple bond
Wedge bond

Hash bond
Mercury (I) Bromide
Mercury (I) Bromide is a chemical compound with the chemical formula Hg2Br2. It changes color from white to yellow when heated and fluoresces salmon-colored when exposed to ultraviolet light. It has applications in acoustic-optical devices.
Mercury(I) Bromide
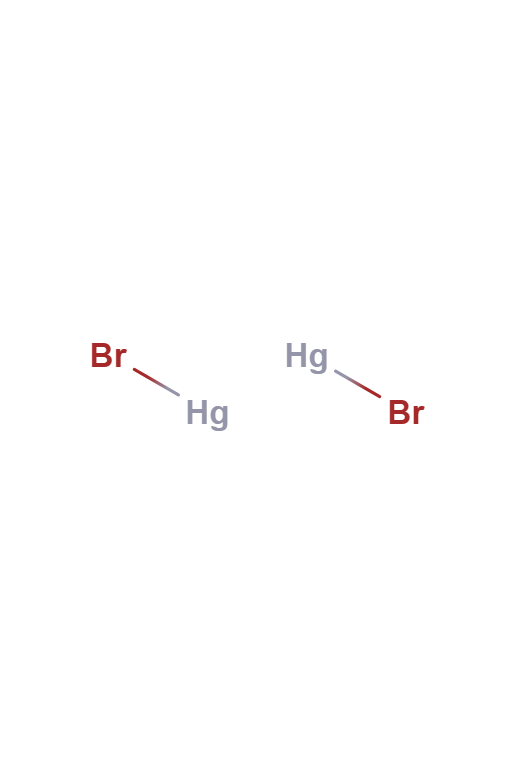
 Single bond
Single bond
Double bond
 Triple bond
Triple bond
Wedge bond

Hash bond
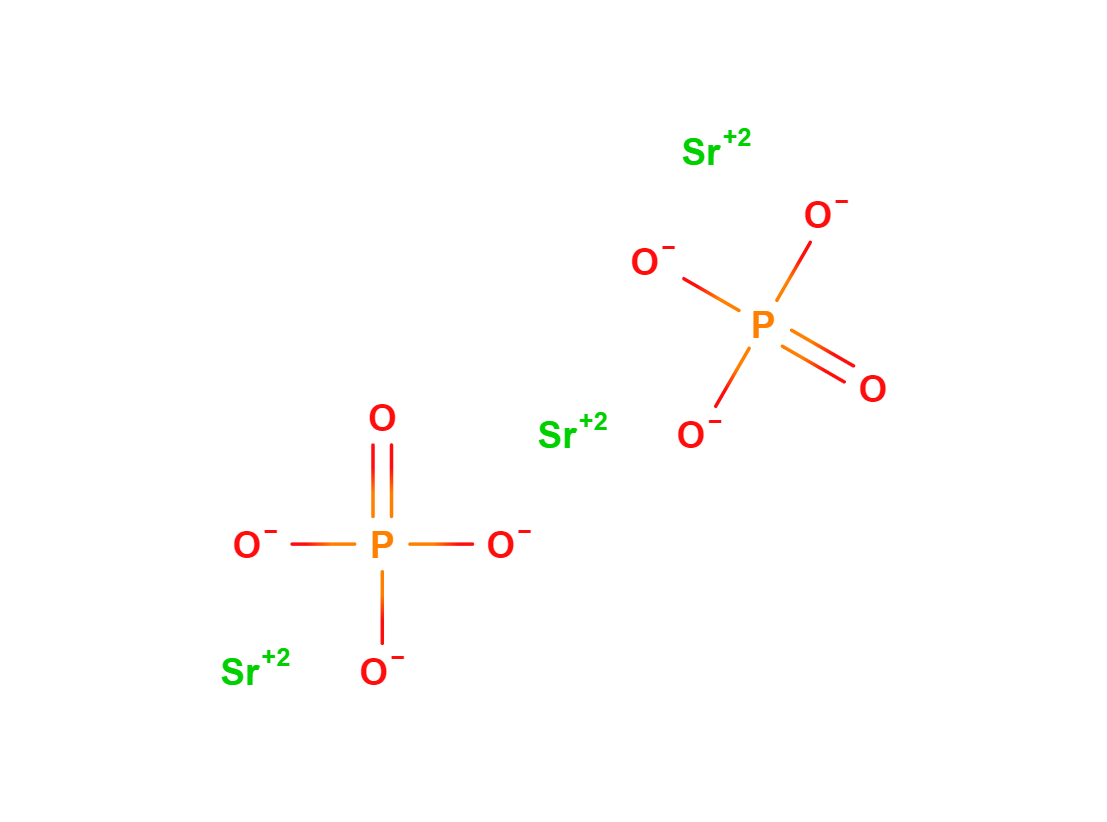
Strontium Phosphate
Strontium Phosphate is a chemical compound with formula O8P2Sr3. It is a crystalline substance used in medicine and industry.
Strontium Phosphate
 Single bond
Single bond
Double bond
 Triple bond
Triple bond
Wedge bond

Hash bond
Giants of science
“If I have seen further, it is by standing on the shoulders of giants”
Isaac Newton

Dmitri Ivánovich Mendeleev
–

Antoine-Laurent de Lavoisier
–
Become a giant


Introduction to Solid State Chemistry



Pre-University Chemistry



Preparing for CLEP Chemistry: Part 1



Big Bang and the Origin of Chemical Elements

Professional development for Educators


Unlocking the Power of Generative AI with ChatGPT for Higher Education