The online states of matter simulations on this page visualize the behavior of matter at the molecular level in the three most common states of matter: solid, liquid and gas.
The states of matter are the forms in which matter appears and can be classified. The most common states are solid, liquid and gas, but there are also other, less well-known states, such as plasma and the Bose-Einstein condensate.
In the solid state, the particles that make up matter are tightly bound together and have an ordered structure. The atoms, ions or molecules in a solid are held in fixed positions and have only vibrational motions. Solids have a definite shape and volume.
In the liquid state, the particles have a less ordered structure and are farther apart than in the solid state. The particles can move freely, although they are still in contact with each other. Liquids have a defined volume, but do not have a defined shape and take the shape of the container that holds them.
In the gaseous state, the particles are widely separated and move in a disorderly and rapid manner. They have no fixed structure and occupy the entire available volume of the container in which they are found. Gases have no definite shape and expand to completely fill the available space.
Plasma is a state of matter found at high temperatures or under high energy conditions. In plasma, the atoms are ionized, i.e., the electrons are separated from the nuclei. Plasma is highly electrically conductive and is present in phenomena such as lightning and in the interior of stars.
The Bose-Einstein condensate is an exotic state that occurs at temperatures very close to absolute zero. In this state, a large number of particles behave as if they were a single particle, giving rise to collective quantum phenomena.
These different states of matter have different properties and can be transformed from one to another by changes in temperature and pressure. Understanding the states of matter is fundamental to physics and chemistry, as they affect many properties and processes in our everyday environment and in the universe in general.
Explore the exciting STEM world with our free, online simulations and accompanying companion courses! With them you'll be able to experience and learn hands-on. Take this opportunity to immerse yourself in virtual experiences while advancing your education - awaken your scientific curiosity and discover all that the STEM world has to offer!
- Solid
- Liquid
- Gas
- States
Molecules in a solid
Molecules in a solid can vibrate but without changing their position. What is the relationship between temperature and the kinetic energy of the molecules? Change the temperature to find out.
Molecules in a liquid
Las moléculas de un líquido pueden cambiar su posición, pero el volumen del líquido no cambia.
Molecules in a gas
The molecules of a gas move at high speed in any direction. What determines the volume of the gas?
States of Matter
Look at the different types of molecules that make up a solid, liquid, or gas. Add or remove heat and see the phase change. Change the temperature or volume of a container and see a pressure-temperature diagram change in real time. Relate the interaction potential of forces between molecules.
File
Chemistry courses


Pre-University Chemistry



Preparing for CLEP Chemistry: Part 1



Big Bang and the Origin of Chemical Elements



Thermodynamics and Phase Equilibria



Thermodynamics



The Basics of Transport Phenomena



Entropy and Equilibria

Other courses


Electricity and Magnetism, Part 1



Unconventional Reservoir Geomechanics



The History of Ancient Environments, Climate, and Life



AP® Physics 1 – Part 1: Linear Motion



Introduction to Water and Climate



Electricity & Magnetism, Part 2



Hypersonics – from Shock Waves to Scramjets

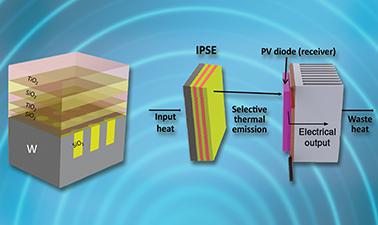

Nanophotonic Modeling